Your Time has Finished
Loading...
GAMSAT Section III Chemistry – Part 1
Your Score: %
Average Score of All Users:
You performed better than of students
Section Breakdown
| Your Score | Average of all Users | Percentile | |
|---|---|---|---|
| Chemistry - Part 1 |
Chemistry - Part 1
Your score:
Average score:
You performed better than of students
Speed as well as accuracy is important in this section. Work quickly, or you might not finish the paper. There are no penalties for incorrect responses, only marks for correct answers, so you should attempt all questions. Each question is worth one mark.
You must complete the answers within the time limit. Calculators are NOT permitted.
Good Luck!
ELECTROLYSIS AND ELECTROCHEMICAL CELLS
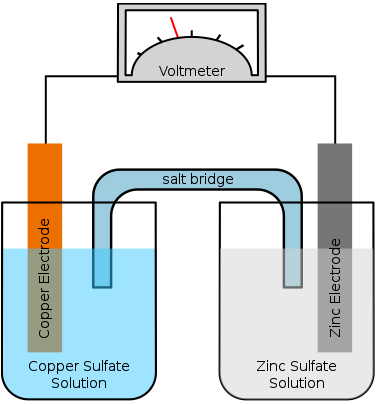
A salt bridge (figure 1) is essential in maintaining electrical neutrality in an electrochemical cell. Without a salt bridge, the two different compartments will accumulate the opposite charges and limit any further reaction.
2Ag⁺(aq) + Cu(s) →2Ag(s) + Cu²⁺(aq)
Explanation
The correct answer is D.
KCl would be a good choice normally because it does not react with any of the chemicals used in the cell, and the anion and cation have similar conductivity, and hence similar migratory speed. In this case, since one of the ions is silver and silver chloride is insoluble, a KCl bridge is not appropriate.